o3 lewis structure
Because of this you cant have 2 pairs of double bonds since. Web Resonating structures of Ozone.
 |
| Chemistry Net Simple Method For Writing Lewis Structures Ozone O3 And Carbonate Co3 2 |
Ozone o3 is a triatomic molecule with 1168 bond angles and a bent electronic geometry and trigonal planar molecular geometry.
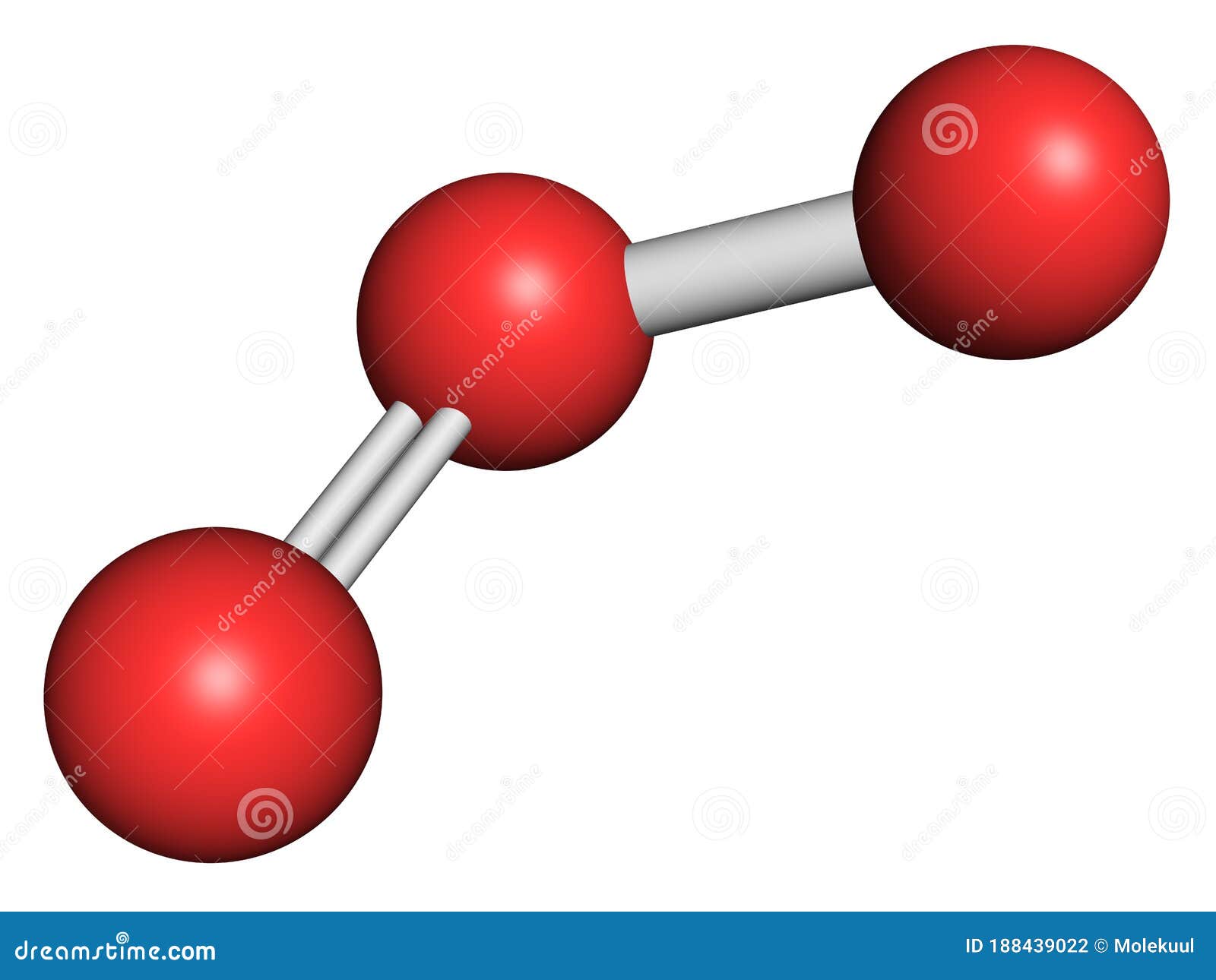
. O 3 has 18 valence electrons 3 6e- 18e-. In an o3 lewis. Web Learn Exam Concepts on Embibe. Web 4 rows Step 1.
Calculate the total number of valence electrons. We can draw O3 different ways. We can easily find the solution to this with one. We now need to determine the central atom.
For the Lewis Structure for O 3 it is possible to draw it two different says slightly different but still important. Web So the total number of sigma bonds in a single SO3 molecule is three and the total number of lone pairs are 0 confirm with the Lewis structure. Check the stability of lewis structure. How can we do so.
Web Heres how you can draw the O 3 lewis structure step by step. The stability of lewis structure. The ideal electron geometry of O 3 is trigonal planar. Web Xeo 3 Lewis Structure Octet Rule.
First we can draw it with a double bond on the left and a single bond on the right. O3 covers the whole. Web Bentuk struktur O3 lewis. In XeO 3 geometry xenon holds one lone pair and there are.
Were using all 18 valence electrons for. When atoms have 8 electrons in their valence shell they are said to have an octet. Now you have come to the final step in which you have to check the stability of lewis structure of O3. Web Drawing the Lewis Structure for O 3.
Web In the above structure you can see that the central atom sulfur forms an octet. In the O3 Lewis structure there is a double bond between the central oxygen atom and one lateral oxygen atom. Web Postby AuraCruzHeredia_1L Tue Oct 27 2015 1130 pm. Web Lewis Structure Step 1.
And the outside atoms oxygens also form an octet. Ozone O 3 is an oxygen allotrope composed of three oxygen atoms. Hence the octet rule is. It can determine reactivity polarity color attraction.
Ozon o3 adalah molekul triatomik dengan sudut ikatan 1168 dan geometri elektronik bengkok dan geometri molekul trigonal planar. The Lewis dot structure of O 3 has two equivalent. Web Molecular geometry is the three-dimensional structure of the atoms which helps in the constitution of a molecule. Web O3 Lewis Structure- Key Points.
In Lewis Structures a line is used to represent the bonding electrons between two combining atoms. Ozone has one double bond and one single bond in its Lewis structure. Web O3 lewis structure shape. Here the given molecule is O3 ozone.
The initial step towards forming this structure is to find out the total number of valence electrons. Web Ozone O 3 molecule has a bent or V-shape and molecular geometry. So Number of hybrid.
 |
| Answered A Lewis Structure For Ozone O3 Is Bartleby |
 |
| Why Can T The O3 Structure Be This R Chemhelp |
 |
| Chem1902 Oxygen |
 |
| O3 Lewis Structure Drawings Hybridization Shape Charges Pair And Detailed Facts Lambda Geeks |
 |
| Atomic Structures Exemplified By Ozone That Do Not Neatly Fit Lewis Structures Are Called A Covalent Bonds B Polyatomic Molecules C Resonance Forms D Triple Bonds Homework Study Com |
Posting Komentar untuk "o3 lewis structure"